
We’ve married together mechanical, textile and biomedical engineering staff with technical veterans who’ve been creating novel textile structures for decades; and backed them with state-of-the-art equipment, unmatched in the industry.
![]()
How We Do It
We’ve married together mechanical, textile and biomedical engineering staff with technical veterans who’ve been creating novel textile structures for decades; and backed them with state-of-the-art equipment, unmatched in the industry.
![]()
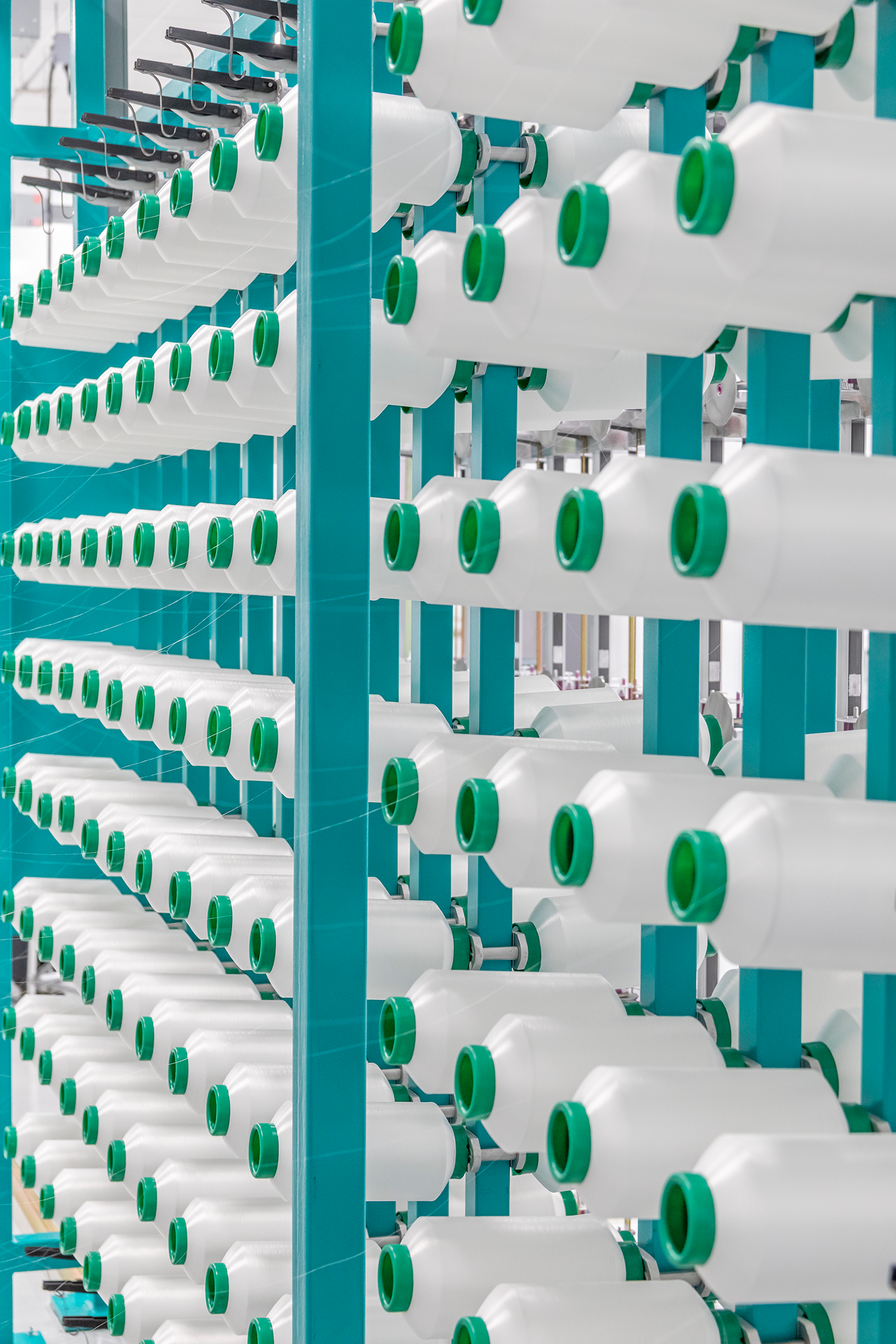
We have made a significant investment in advancing our capabilities for biomedical textiles.
Backed by the state-of-the-art resources of the new facility, our engineers are able to better support you to help solve the next generation of medical device problems.

We have made a significant investment in advancing our capabilities for biomedical textiles.
Backed by the state-of-the-art resources of the new facility, our engineers are able to better support you to help solve the next generation of medical device problems.
Our Process
Our four-phased design and development process is designed to demonstrate compliance to ISO 13485:2016.
PHASE 1
PHASE 1
Concept and Feasibility
Our goal in the Concept and Feasibility phase is to rapidly transform your design inputs into a functional prototype. This often requires an iterative approach, where our team balances the various physical and mechanical attributes of a fabric structure with an optimal device design.
Our speed and agility during this process is vital to assist you in reaching a key developmental milestone as rapidly as possible.
We’ve prioritized this aspect of our Design and Development process by assembling a team from across a wide range of engineering and technical disciplines. We’ve married together mechanical, textile and biomedical engineering staff with technical veterans who’ve been creating novel textile structures for decades. We’ve enabled this team to be successful on your behalf by providing them with state-of-the-art equipment, unmatched in the industry.

PHASE 2
PHASE 2
Design & Development
We enter the Design & Development phase by formally entering ISO 13485:2016 design control and beginning the process of establishing a robust and repeatable manufacturing process for your product. Our team uses this phase of development to work closely with you to build the requisite documentation for your design history file for your regulatory filings. Key among these are protocols and documents for the following:
- EIQ/EOQ- Equipment Installation Qualification and Equipment Operational Qualification – Cortland Biomedical is able to offer you access to the installation and qualification protocols that have been executed to start-up our equipment in our ISO Class 7 & 8 clean rooms.
- EFS/FIM Early Feasibility Study/ First In Man study- Often times with certain medical devices, an early stage of the medical device is required to prove the concept is sound and the product works as intended. Our team will work closely to streamline our processes to pilot scale to support small-scale manufacture of components for these important clinical trials.
- pFMEA- process Failure Mode Effects Analysis- A critical tool that we deploy to quantify the risks inherent in the process and take meaningful action steps to mitigate these risks. We encourage full transparency during this process and invite you to participate in the creation and review of this living document as a means of understanding the full scope of the product.
- MSA- Measurement Systems Analysis- It is typical for us to participate in the development of a novel test method for measuring a key aspect of our product and sharing that data with you. Conducting a MSA protocol is a typical method to correlate our methods between your site and ours and to ensure the repeatability and reliability of our methods and instruments.
- DOE- Design of Experiments-We will routinely use a range of design tools, including DOE, to isolate the factors that can influence the design.
- Process Characterization- We are accustomed to working with you to help set appropriate specifications for your biomedical components, including specification nominals and tolerances. We do this by executing process optimization protocols designed to make statistically sound decisions during this critical phase of development.

PHASE 3
PHASE 3
Scale-Up & Transfer
During the Scale-Up and Transfer phase of our process, we are tasked with ensuring that the manufacturing prototype that worked for small-scale production is able to accommodate the volume manufacturing to ensure your commercial success.
- OQ- Operational Qualification- A key aspect of process validation is to challenge the upper and lower limits of each of the sub-processes that we have designed for our client’s product. We write and execute protocols to ensure that we can confidently manufacture our biomedical textile component within the set tolerances at the various limits of our process range.
- PQ- Performance Qualification- The most important portion of our verification and validation activities is Performance Qualification. We demonstrate stable manufacturing processes to you by delivering protocols and statistical data demonstrating our products can be made over and over with no deviation.

PHASE 4
PHASE 4
Commercial Launch
The successful completion of PQ completes the handshake between our development and operations teams and represents the commercial launch of our product to support your medical device. Our goal is to support you through your product launch and ramp up in volume. During this phase, our work is not complete. We deploy our manufacturing engineering teams to work on continuous improvement items to drive costs down while delivering on our commitment to quality.
Our team remains committed to our designs and your products and meets frequently with our operations teams to stay current with the challenges and opportunities for the next generation of the product.




